European Youth Face Uneven Access to Innovative Cancer Therapies
Every year in Europe, thousands of parents are faced with the most difficult diagnosis possible: cancer in a child. Although modern medicine offers increasingly advanced therapies, the OCEAN project highlights inequalities between European countries when it comes to access to clinical trials.
New data from the OCEAN project have revealed a significant divide in European pediatric oncology. While medicine is advancing at an extraordinary pace, access to its benefits still largely depends on the European country where a child lives.
Scientists involved in the OCEAN project (Organisation of Care & rEsearch for children with cANcer in Europe) aimed to describe the availability of clinical trials for this population, the inequalities between countries, and to propose solutions to reduce these disparities. They emphasize an urgent need to address differences in the availability of clinical trials at both European and national levels in order to advance equity and improve care, research, and access to innovation for all pediatric, adolescent, and young adult patients with cancer in Europe.
The results were published in the study titled “Inequalities in availability of clinical trials for pediatric, adolescent, and young adult patients with cancer in Europe: results from the SIOPE OCEAN project.” The study authors are: Kerstin K. Rauwolf, Teresa de Rojas, Miguel Martins, Maria Otth, Uta Dirksen, Delphine Heenen, Lejla Kameric, Pamela Kearns, Ruth Ladenstein, Cormac Owens, Caroline Queiroz, Richard Sullivan, Carmelo Rizzari, and Gilles Vassal, on behalf of the European Society for Paediatric Oncology (SIOPE).
A warning from the numbers
According to the data, around 35,000 children and young people are diagnosed with cancer in Europe each year. Although survival rates in wealthier countries have exceeded 80 percent, researchers warn that differences in survival between European countries can reach up to 20 percent. Among children older than one year, cancer remains the leading cause of death across the continent. The largest gap is observed in Eastern Europe, particularly for central nervous system (CNS) tumors, sarcomas, and high-risk neuroblastoma. The reasons are not exclusively financial; they also include delayed diagnosis and a lack of specialised centres capable of implementing the latest treatment protocols.
Clinical trials: luxury or standard of care?
In pediatric oncology, clinical trials are considered the “gold standard” of treatment. They are not merely research studies but are often the only way for patients to access the newest, most innovative medicines. “Despite the importance of access to clinical trials for pediatric/AYA patients with cancer, no systematic analyses describing discrepancies across European countries have been published so far. In this report, we present the results of the OCEAN project, specifically describing the national availability of clinical trials for pediatric/AYA patients with cancer across Europe over the last 13 years (2010–2022) as an indicator of access, and we discuss proposals to reduce inequalities,” the study’s authors note.
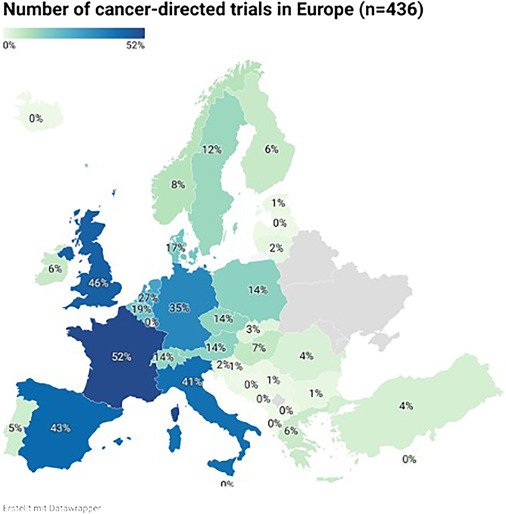
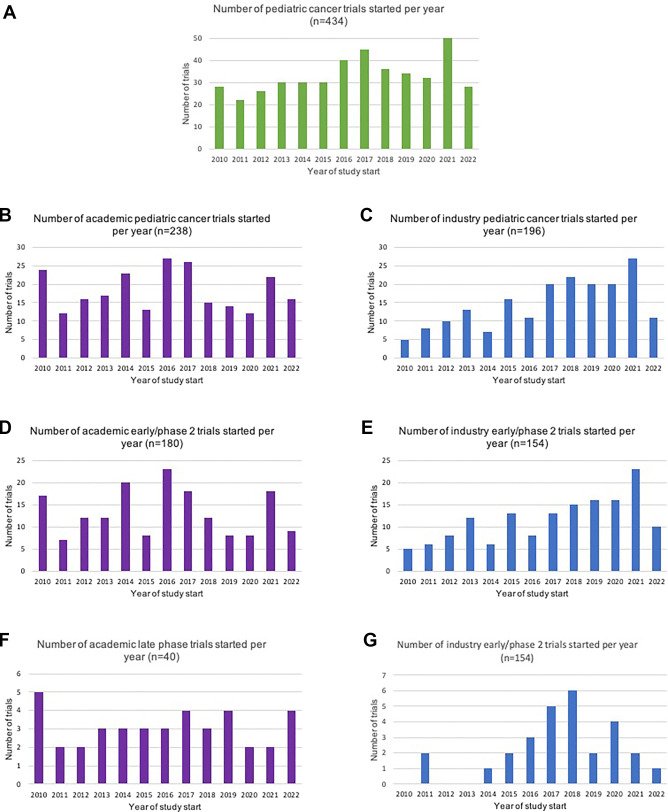
The search of the ClinicalTrials.gov database yielded 2,861 studies, and after removing duplicates and applying a number of other criteria, 436 cancer-focused clinical trials were found. By analysing 436 clinical trials conducted between 2010 and 2022, the researchers found, among other things, that trials investigated novel therapies, such as targeted therapies, immunotherapies, and cell therapies, including CAR T-cells, were more frequently sponsored by industry than by academic trials (184, 93% vs 148, 62%). On the other side, monocentric studies were predominantly sponsored by academia, while intercontinental studies were mostly led by industry.
Age-based exclusion and major differences
One of the most striking problems identified is age-based exclusion. As noted in the study, while half of the pediatric trials allowed inclusion of older adults (>30 years), only 18% of the studied trials are adult trials, lowering their entry age to allow inclusion of adolescents (minimum age 13–18 years).
The geographical divide is even more stark. France, the United Kingdom, and Spain lead in the number of clinical trials. In contrast, countries such as Montenegro, North Macedonia, Malta, Cyprus, and Luxembourg had no pediatric cancer clinical trials during the period analysed. Albania, Bosnia and Herzegovina, and Latvia each had only one available trial.
“Even though survival rates for pediatric oncology patients in Eastern European countries are increasing, there are still major differences, with inferior outcomes observed compared to the other parts of Europe. The lack of health-care resources with limited drug supply, lack of specialized centers with multidisciplinary teams, delayed diagnosis and treatment, poor management of treatment, and drug toxicity are considered as reasons for the gap in survival rates across Europe. In light of their role as the gold standard in integrated childhood cancer care, we believe that access to clinical trials can be a contributing factor for the survival of these patients,” the scientists explained in the study.
Additionally, they recalled that there are calls for proposals and funding opportunities for academic pediatric oncology trials under Horizon Europe, the current EU Framework Programme for Research and Innovation, and the EU4Health programme.
Can the gap be bridged?
The researchers warn that significantly fewer clinical trials were launched in Eastern Europe between 2010 and 2022 than in Northern and Western Europe. However, they stress that the goal is not to have every clinical trial conducted in every European country. According to their recommendations, randomized trials should be available in as many countries as possible.
The emphasis is also placed on the costs of cross-border participation in industry-sponsored trials, which are usually covered by the industry; such a funding model does not exist for academic trials, which further complicates access for patients. The recommendations include, among other things, improving equal access to clinical trials and innovative therapies for patients across Europe, enhancing cross-border networking with academic and EU-supported consortia, ensuring effective use of structural funds at national and/or EU level, increasing the number of clinical trial experts with applied knowledge, improving patient referral pathways not only through funding, but also through the transfer of knowledge and skills to countries with more limited access to innovation.
The recommendations addressed to the European Union also refer to ensuring that childhood cancer is consistently and clearly defined as a priority within relevant EU policies and budgetary programmes related to research, innovation and health, as well as to integrating clinical trials for rare and complex diseases, in particular early-phase trials, into the application of EU cross-border healthcare legislation, currently governed by Directive 2011/24/EU and Regulations 883/2004 and 987/2009.
The recommendations addressed to European countries relate to the need to include a clearly defined section on childhood cancer in National Cancer Control Plans or equivalent strategic documents, covering all stages of care as well as cross-cutting areas, in particular, access to clinical trials and innovation. They also include simplifying logistical procedures and administrative requirements for clinicians, researchers, and patients with regard to cross-border access to clinical trials in Europe and participation in international studies, as well as considering the use of national and EU structural funds to strengthen research capacity and local infrastructure in pediatric oncology.
The OCEAN project shows that there are major disparities in access to clinical trials and innovative therapies for pediatric and AYA cancer patients across Europe. Every year, around 6,000 children in Europe die from cancer, further highlighting the urgent need for more equitable and accessible treatment, where national borders should not be barriers to accessing the best therapies.
Image: The OCEAN Project, SIOPE.EU
Funding: This work has been performed as part of WP6 on inequalities in cancer research of the 4.UNCAN.eu Coordination and Support Action (#101069496) funded by the European Union and has been supported by Zoé4Life (MO).


